Research Topics
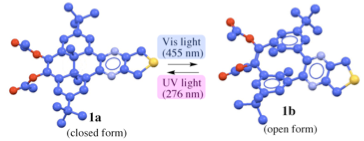
Sawada, Tsuyoshi ; Kurokia, Mizue; Ogawaa, Tomoya; Shimojoa, Kentaro; Chifukua, Kazufumi; Iharaa Hirotaka. Dihydropyrene annelated with dihydrothieno[3.4-b]pyrazine: synthesis and photoswitching property, Tetrahedron Letter (2010), 51(31), 4033-4036.
In this study, 9,10-diacetoxyl-2,7-di-tert-butyl-trans-10b,10c-dimethyl-10b,10c-dihydropyrene annelated with a dihydrothieno[3.4-b]pyrazine unit (1a) was prepared, for the first time, from 5,13-di-tert-butyl-8,16-dimethyl-1,2,9,10-tetrahydroxy[2.2]metacyclophane and 3,4-diaminothiophene in two steps. The photoisomerization property of 1 was investigated by UV and 1H NMR spectroscopies, and the quantitative isomerization between the more stable dihydropyrene (DHP) form and the less stable metacyclophane-diene (MCPD) form was observed. A thermally induced return reaction from the MCPD to the DHP form was examined at various temperatures, and the reaction rate was 0.0049 min−1 at 45 °C, which is slower than that of the parent MCPD.
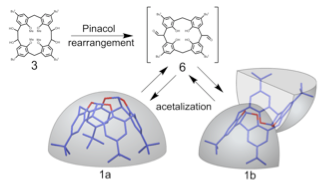
Sawada, Tsuyoshi; Hongo, Takuya; Matsuo, Nami; Konishi, Masakazu; Kawaguchi, Tsutomu; Ihara, Hirotaka.
Hemisphere-shaped calixarenes and their analogs: synthesis, structure, and chiral recognition ability.
Tetrahedron (2011), 67(25), 4716-4722.
The hemisphere-shaped calixarene 1a and its split-hemisphere-shaped isomer 1b were synthesized from [2.1.2.1]metacyclophane (MCP) 3 by pinacol rearrangement and subsequent intramolecular acetalization. Their structures were revealed by X-ray crystallography and 1H-NMR spectroscopy. The temperature-dependence of the intramolecular acetalization to provide 1a and 1b was examined. The results indicated that 1a is the dominant product at high temperatures, and the values of ΔH˚and ΔS˚ were estimated to be - 18.3 ± 0.37 kJ/mol, - 59.1 ± 1.12 kJ mol-1・K-1, respectively. The dinitro derivative 7 and tetranitro derivatives 8 were obtained by ipso-nitration at the upper rims of 1a. The optical resolution and chiral recognition ability of racemic mixture 7 were investigated by HPLC systems.
Sawada, Tsuyoshi; Kihara, Takao; Fujikawa, Yuya; Narazaki, Yu
”Synthesis and photochromic properties of quinoxalino[e]-annelated dimethyldihydropyrene with planar chirality”
Tetrahedron Letters (2013), 54(45), 5963-5966.
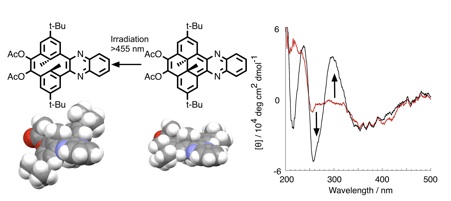
9,10-Diacetyl-2,7-di-tert-butyl-trans-10b,10c-dimethyl-10b, 10c-dihydropyrene annelated with a quinoxaline unit (q-DM-DHP, 3) was prepared from 5,13-di-tert-butyl-8,16-dimethyl [2.2]metacyclophane-1,2,9,10-tetraoxide (2) and o-phenylenediamine in two steps. The photochromic isomers of q-DM-DHP 3, closed form 3a and open form 3b, were separated by reverse-phase HPLC, and their isolated UV-visible spectra were estimated. The chiral HPLC analyses of q-DM-DHP 3 indicated their planar chirality, and their CD spectra were measured. The photochromic properties and thermodynamic properties of 3 were also discussed based on their UV-visible, 1H-NMR, CD, and fluorescence spectra.
